Two CD19-specific CARs approved by US FDA in 2017 have induced prominent responses in patients with refractory leukemia and lymphomas. However, Grade 3/4 CRS and ICANS were reported in proportion patients and required ICU transfer. Scientists are continuously investigating new strategies to augment CAR-T cell function and simultaneously minimize treatment-related toxicities.
Recent Progress
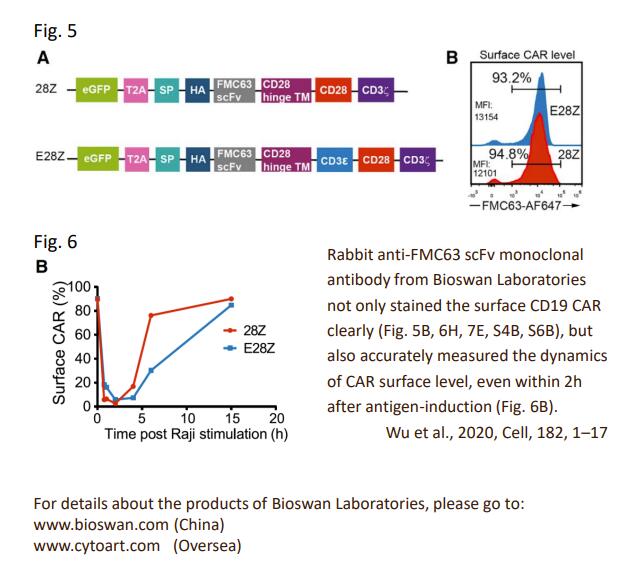
Recently, Wei Wu and his colleagues suggested on Cell Multiple Signaling Roles of CD3ε and Its Application in CAR-T Cell Therapy that increasing CD3 diversity may represent a novel strategy for next-generation CAR.
In nature, it is not typically to notice excessive cytokine release in TCR-mediated response. The entire TCR-CD3 complex contains 10 ITAMs with 20 tyrosine phosphorylation sites to deliver diverse signals in response to different antigen stimulation. Each CD3 ITAM has a distinctive sequence. Will each of them have a specific role? As of today, the existing CAR constructs only contain the activating CD3ζ chain to mediate antigen signaling. Can we utilize nature mechanism to improve CARs?
Wei Wu and his colleagues reported that TCR is indeed self-restrained signaling machinery. It contains both activating and inhibitory motifs. CD3ε might be the key, as it is a built-in multifunctional signal tuner. When incorporating CD3ε cytoplasmic domain into a second-generation CD19.28Z CAR, they observed improved antitumor activity of CAR-T cells, whereas reduced multiple cytokine productions. In vivo experiments even suggested promotion of persistence.
To me, these are important findings for 28Z CAR improvement, as in comparison to 4-1BBZ CAR, it is deemed to have less superiority of longevity in patients and be prone to onset tonic T cell signaling. Clinical studies verifying this CAR-design strategy is worth longing for.
In summary, we show that TCR is a self-restrained signaling machinery that contains both activating and inhibitory signaling modules embedded in diverse CD3 signaling subunits. Increasing CD3 diversity of CAR, i.e., incorporation of CD3ε cytoplasmic domain, yielded a CAR with improved signaling profile and tumor control
Our Approach
Last, but not least, BioSwan Laboratories and Cytoart are honored that our product, the anti-CD19 FMC63 scFv idiotype antibody, could contribute to this meaningful study.