Immune checkpoint inhibitor was undoubtedly a breakthrough in cancer therapy. Since FDA approved Pembrolizumab and Nivolumab for melanoma in 2014, PD-1/PD-L1 blockade has been proven to be effective in multiple cancers including lung, head and neck, kidney, breast, bladder cancer, and lymphoma.
 Cover of Nature, Volume 515 Number 7528, 27 November 2014. This issue of Nature featured five papers reflecting the intense interest in the targeting of immune checkpoints as cancer therapy after PD-1/PD-L1 axis inhibitors were approved by FDA.
Cover of Nature, Volume 515 Number 7528, 27 November 2014. This issue of Nature featured five papers reflecting the intense interest in the targeting of immune checkpoints as cancer therapy after PD-1/PD-L1 axis inhibitors were approved by FDA.
In relation to the living drug CAR T cells, interrupting the PD-1/PD-L1 pathway was suggested to be critical in treating PD-L1+ tumour. For instance, administration of Pembrolizumab to a patient with refractory DLBCL failing to anti-CD19 CAR T treatment could re-constitute antitumor response and resulted in clinical significances: an expansion ofCART19 cells, and decreased co-expression of PD-1 and Eomes by CART19 cells (Chong EA, et al., Blood. 2017 Feb 23;129(8):1039-1041).
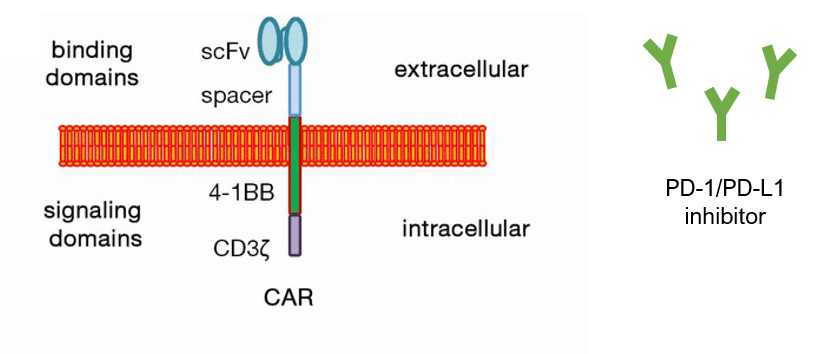
with CAR T cells and immune checkpoint inhibitor agents, genetically engineering T cells represents another promising strategy. The constitutive anti-PD-1 can be either built-in into a CAR structure or be secretive and delivered by the CAR.
Secretion of anti-PD-1 scFv by CAR T cells has been shown to further “armoured” CAR T-cell efficacy and persistence via different mechanisms. Si Li and his colleagues observed that “armoured” CAR T cells are more expandable and functional in the face of a hostile tumour microenvironment than their parental CAR T cells (Li S, et al., Clin Cancer Res. 2017 Nov 15;23(22):6982-6992). To my knowledge, this strategy is currently under clinical investigation by several Biotech companies. I am looking forward to their clinical outcomes.
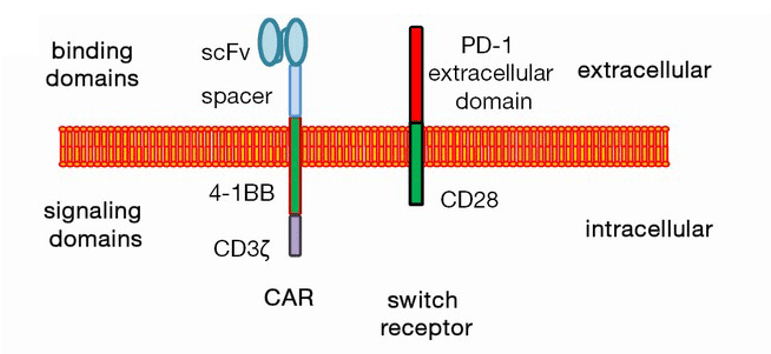
Promoted by UPenn’s pre-clinical work, Hui Liu, et al., explored the clinical application of PD-1/CD28 chimeric switch-receptor in treatment of PD-L1+ R/R large B-cell lymphoma with anti-CD19 CAR T cells (Liu H, Clin Cancer Res. 2020 Oct 7:clincanres.1457.2020). Recently, they reported that among total of 17 adult patients, 10 patients had objective response (58.8%), 7 complete remission (41.2%). It is encouraging, especially when we consider those were heavily treated patients, like Patient 9 who achieved CR was previously refractory to four lines of immnuno-chemotherapy. Also, remarkably, there was no Grade 3 or 4 CRS and neurological toxicity observed.
After all, which treatment modality will prevail over the other? Whether it will be the combination therapy, build-in approach or secretion strategy in tumour microenvironment? There is no telling as of today, but exciting to wait and see more clinical trials with larger cohorts.
Our Approach
In this recent report on applying CD19-PD-1/CD28-CAR T cells in the clinic setting, Hui Liu et al., performed a comprehensive characterization of those CAR T cells. Using flow cytometric analysis, in combination with BioSwan rabbit-anti-mouse FMC63 scFv idiotype antibody to accurately define CD19-CAR positive T cell population, they demonstrated that CD19-PD-1/CD28-CAR T cells displayed a distinguished phenotype and cytokine profile: reduced exhaustion, less senescent and more prone to generate T-cell memory in comparison to the parental T cells.
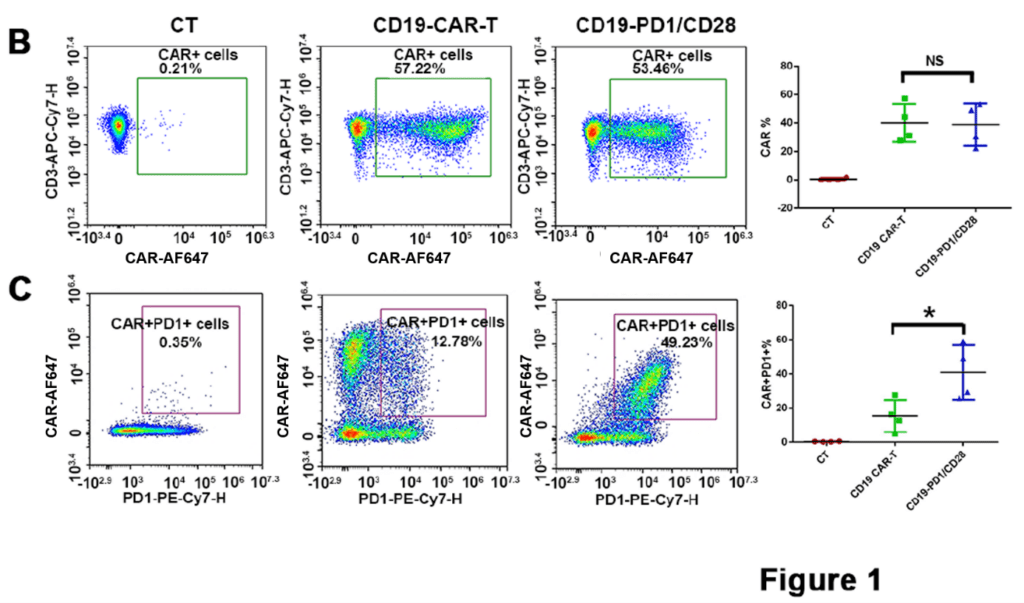
BioSwan Laboratories is honored our product, the anti-CD19 FMC63 scFv idiotype antibody, could contribute to this meaningful study.

