Adaptor CAR platform advances
It almost feels like a Christmas gift for CAR-T community even though December is still ten days to go. On November 19, 2020, Umoja Biopharma launched with $53 million in series A financing. There came a thrill in our hearts when studying their technology platform. Three tricks are integrated: 1) engineer patient T cells in vivo, 2) tune the power of the living drug by an FDA approved medicine, and 3) flag the tumor like switching on the light in the dark for equipped T cells. It is a unification of three approaches developed by scientists at Seattle Children’s and Purdue University, who gave the name “Umoja” – “unity” in Swahili. To us, Umoja’s platform falls into the “adaptor CAR” family, and they are not the first one who thought of employing it.
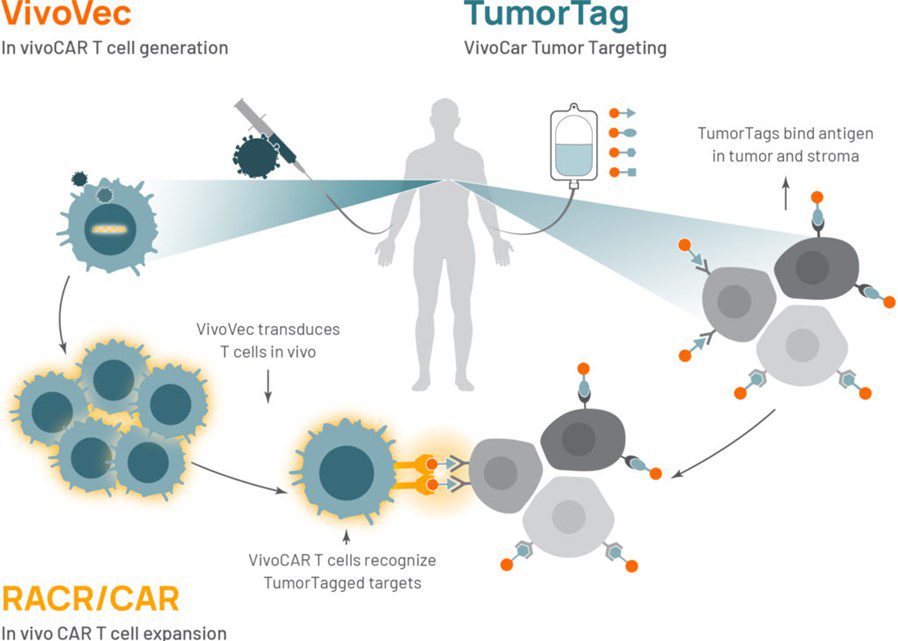
Source: https://www.umoja-biopharma.com/platforms/
In May of this year, Claudia Arndt and her German colleagues published a review on adaptor CAR designs and on-going clinical trials. Much attention has already been paid to adaptor CAR platforms. They provide promising solutions to the major problems of conventional CAR therapy, such as toxicity and antigen escape.
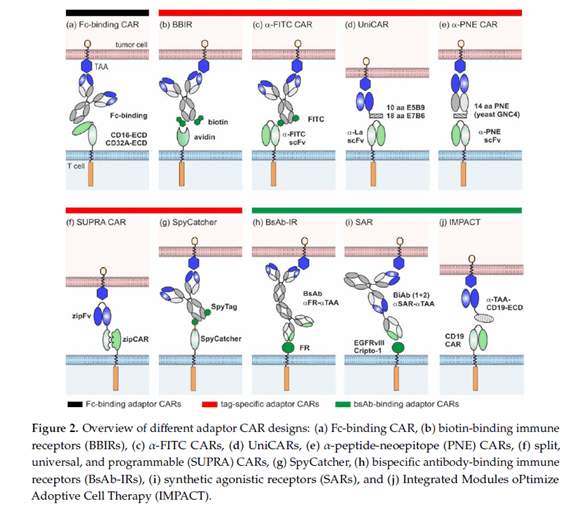
Source: Adaptor CAR Platforms—Next Generation of T Cell-Based Cancer Immunotherapy
But why are we at Cytoart excited about this case? First, it is an in vivo-based universal CAR research, which comprises three incorporative approaches. We have discovered many mechanisms of our immune system. We have employed our ever-growing knowledge timely to engineer our immune cells. Together we can go far! Every novel technology helps to kill tumor cells precisely and effectively. But what if we have a multipronged yet simplified platform? Will we get closer to our dream of making cancer history? Umoja’s platform has presented us with a promising solution. It opens a gate to manipulate cells in vivo in a controlled and switchable manner. Their inherent advantages will allow easy implementation of this treatment in hospitals. Eventually, there will be wide access for cancer patients. Cytoart, as a leader in CAR-T detection and clinical monitoring, welcome any breakthrough in this exciting field!
Umoja plans to use the series A funds to focus on two types of cancer which have limited treatment options: blood cancer and pediatric osteogenic sarcoma. Andy Scharenberg, M.D., the co-founder of Umoja expects to start a clinical trial in the second half of next year. Investors have deep trust in Umoja’s technology experience. CAR-T community is curious how the FDA would challenge these novel tools about their applicability as well.

