excelling in our industry
Who We Are
Cytoart is a leading provider of cell therapy solutions, specializing in CAR-T diagnostics, antibody development, and first-in-human investigator-initiated trials (IITs). As an innovator in IVD solutions for cell therapy, we are transforming how CAR-T and other advanced therapies are monitored and optimized.
We pioneered the world’s first commercial anti-idiotype antibody for CAR-T detection, setting a new standard for precision in cell therapy monitoring. Our cutting-edge technology platform supports all FDA-approved CAR-T therapies, providing researchers and clinicians with highly specific and reliable detection tools.
Beyond antibodies, Cytoart offers comprehensive cell therapy services, including first-in-human IIT support, custom assay development, and analytical solutions to accelerate the adoption of next-generation immunotherapies such as CAR-T, TCR-T, and NK cell therapies.
With a strong commitment to innovation, Cytoart continues to provide state-of-the-art technologies and expert services to pharma companies, CROs, and academic institutions worldwide.
Our Promise to You: Reliability and Predictability in Changing Times
Dear Valued Customer,
Thank you for your continued trust in Cytoart. As global trade dynamics continue to evolve, we want to reassure you of two things that remain constant: our commitment to uninterrupted product supply and our responsible approach to pricing.
Reliable Supply, Unwavering Commitment
At Cytoart, we understand that supply chain consistency is critical to your operations. Despite external challenges, we have maintained a stable logistics network and continue to fulfill orders without disruption. We remain fully committed to providing you with the same high-quality products—on time and as expected.
Cautious, Transparent Pricing Strategy
We are actively monitoring the evolving tariff and trade policy environment and have adopted a cautious, adaptive pricing strategy. Most importantly, we want to emphasize that no pricing changes will occur without at least three months’ advance notice. Our goal is to give you the predictability and planning time you need.
Our customers are the cornerstone of our growth. In times of uncertainty, we believe that adaptability, transparency, and collaboration are essential. Whether you’re using our products for research, clinical monitoring, or early-stage trials, you can count on us for consistent, dependable support.
If you have any questions or need further information, please don’t hesitate to reach out. We’re always here to support you.
We look forward to continuing our journey together.
Warm regards,
The Cytoart Team
the highest sensitivity for CAR T-Cell detection in the market
Our Products
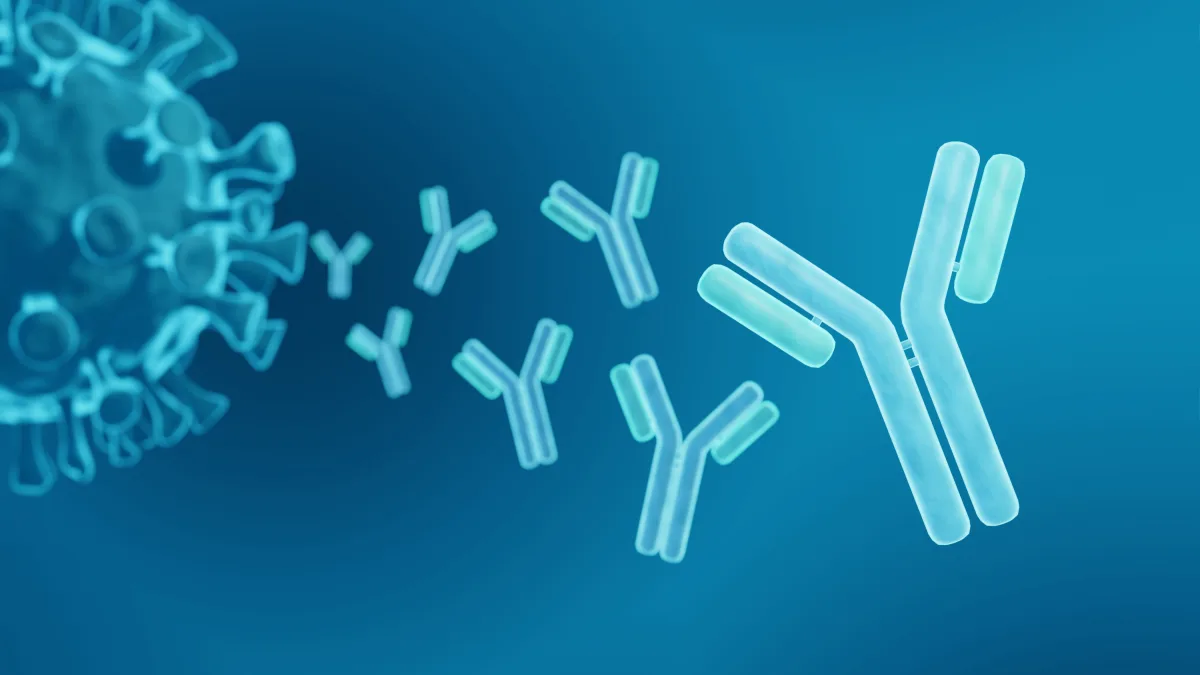
At CytoArt, we are proud to introduce a groundbreaking leap in the realm of immunotherapy and diagnostic research: the first commercially available anti-idiotypic CAR detection antibodies. Our pioneering product stands at the forefront of scientific innovation, offering an unparalleled level of specificity and sensitivity that sets a new standard in the industry.
Our antibodies are meticulously engineered to excel in a diverse range of applications, including Flow Cytometry and PCR, catering to the critical needs of Ligand Binding, Pharmacokinetics (PK), Anti-Drug Antibody (ADA)/Neutralizing Antibody (Nab) Analysis assays. This versatility extends to the domain of long-term in-vivo CAR monitoring, underscoring our commitment to supporting the ongoing needs of both research and clinical diagnostics.
At CytoArt, we believe in empowering our clients with the tools they need to push the boundaries of what’s possible in cancer treatment and diagnostic methodologies. Our products are not just tools; they are gateways to new discoveries and innovations that will lead the way in the fight against disease.
your questions, Answered
FAQ’s
Have some questions? We’ve compiled a list of the most commonly asked questions around our products.
data & Information
Latest Articles
Welcome to the Cytoart blog, where curiosity meets discovery and knowledge reigns.
Exciting News for CARVYKTI®!
Johnson & Johnson has just released their 2024 Annual Report, showcasing remarkable progress for Carvykti (cilta-cel), the revolutionary CAR T-cell therapy for relapsed or refractory multiple myeloma. 🔹 [...]
Congratulations to the June Lab on their groundbreaking research in T-Cell Engineering!
The Carl June Lab has once again advanced the frontiers of immunotherapy research with their recently published study, "Stem Loop Mediated Transgene Modulation in Human T Cells" featured [...]
The Benefits of Using Anti-Idiotype Antibodies for CAR Detection in CAR T Therapy
Chimeric Antig en Receptor T-cell (CAR T) therapy has revolutionized the treatment of certain hematological malignancies, offering hope for patients with refractory or relapsed cancers. As the use of [...]